WHO issues highest alert for monkeypox outbreak, declares it global health emergency
The World Health Organization has declared the monkeypox outbreak, which has affected nearly 17,000 people in 74 countries, to be a global health emergency; the highest alarm it can sound.
"I have decided that the global monkeypox outbreak represents a public health emergency of international concern," WHO chief Tedros Adhanom Ghebreyesus said at a press conference on Saturday.
He said a committee of experts who met on Thursday was unable to reach a consensus, so it fell to him to decide whether to trigger the highest alert possible.
"WHO's assessment is that the risk of monkeypox is moderate globally and in all regions, except in the European region where we assess the risk as high," he added.
Monkeypox has affected over 16,800 people in 74 countries, according to a tally by the US Centers for Disease Control and Prevention (CDC) published on July 22.
A surge in monkeypox infections has been reported since early May outside the West and Central African countries where the disease has long been endemic.
Tedros has previously expressed concern that stigma and scapegoating could make the outbreak harder to track. On Saturday, he said the outbreak can be stopped with the right strategies in the right groups.
Potential vaccine
On June 23, the WHO convened an emergency committee (EC) of experts to decide if monkeypox constitutes a so-called Public Health Emergency of International Concern (PHEIC) -- the UN health agency's highest alert level.
But a majority advised Tedros that the situation, at that point, had not met the threshold.
The second meeting was called on Thursday with case numbers rising further, where Tedros said he was worried.
"I need your advice in assessing the immediate and mid-term public health implications," Tedros told the meeting, which lasted more than six hours.
A viral infection resembling smallpox and first detected in humans in 1970, monkeypox is less dangerous and contagious than smallpox, which was eradicated in 1980.
Ninety-five percent of cases have been transmitted through sexual activity, according to a study of 528 people in 16 countries published in the New England Journal of Medicine -- the largest research to date.
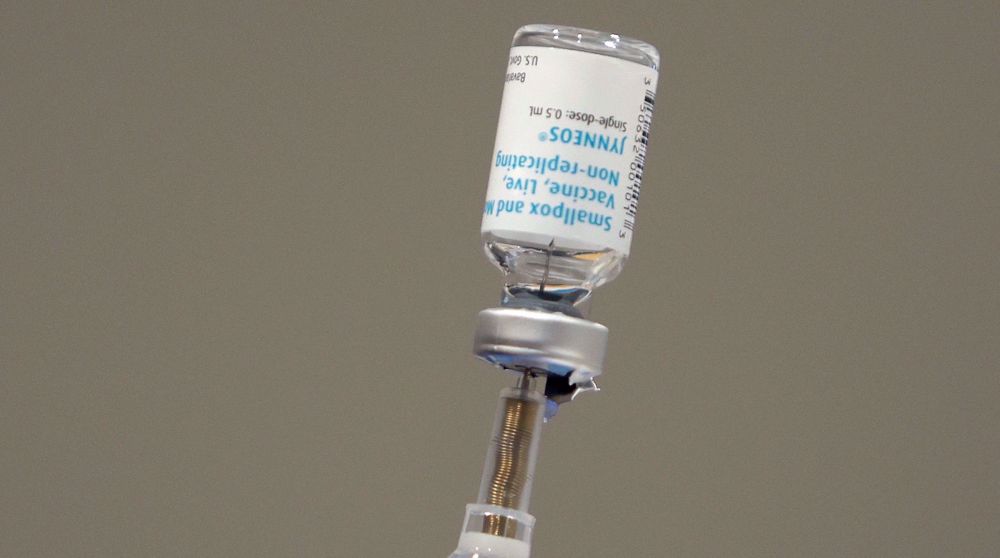
The European Union's drug watchdog on Friday recommended for approval the use of Imvanex, a smallpox vaccine, to treat monkeypox.
Imvanex, developed by Danish drugmaker Bavarian Nordic, has been approved in the EU since 2013 for the prevention of smallpox.
It was also considered a potential vaccine for monkeypox because of the similarity between the monkeypox virus and the smallpox virus.
The first symptoms of monkeypox are fever, headaches, muscle pain and back pain during the course of five days.
Rashes subsequently appear on the face, the palms of hands and soles of the feet, followed by lesions, spots and finally scabs.
(Source: AFP)
From Iraq to Gaza: The great disconnect between British people and rulers
Syria condemns Israel's killing of 36 in 'horrific' strike on Palmyra
Iran rejects UN human rights resolution as politically-motivated, unjustified
US Senate rejects bids to halt military sales to Israel
US veto of Gaza resolution 'license' for more Israeli crimes: Tehran
Iran urges IAEA Governors Board to oppose E3’s anti-Iran resolution
Nov. 20: ‘Axis of Resistance’ operations against Israeli occupation
VIDEO | Iran celebrates National Hero Day, honoring Martyr Qassem Soleimani









 This makes it easy to access the Press TV website
This makes it easy to access the Press TV website