India halts AstraZeneca vaccine exports, EU considers own controls
India puts a halt to exports of the AstraZeneca coronavirus vaccine and the European Union considers it own export bans as the cases of the deadly respiratory rise in their communities.
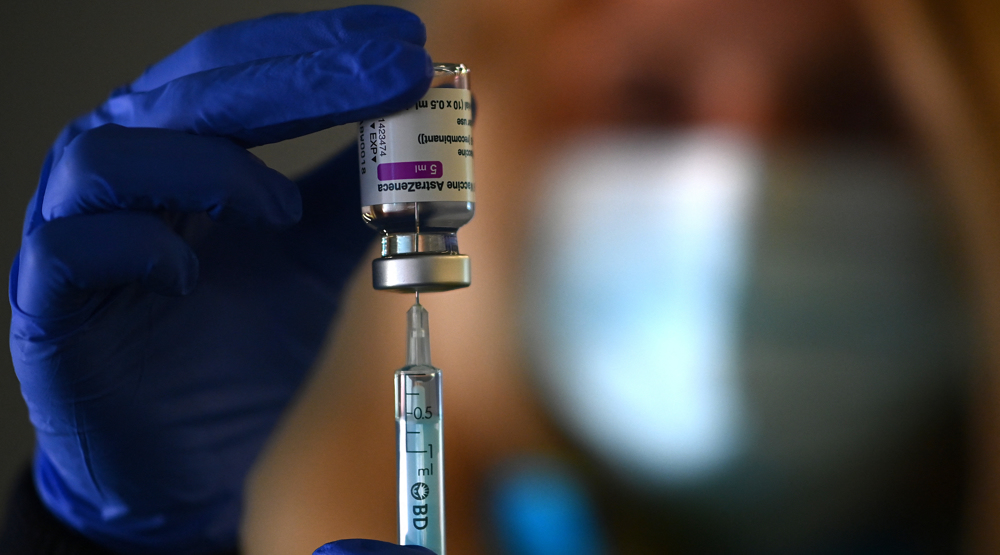
When the United Kingdom started to roll out the vaccine developed by AstraZeneca and the University of Oxford in early January, the drugmaker decided that the majority of its doses would be made in Britain.
Soon after, a dispute over supplies broke out with the European Union which accused the drugmaker of not going to fulfill its contracted deliveries to the bloc.
The EU accuses AstraZeneca of overselling its vaccine and unfairly favoring Britain.
The rollout of the vaccine now faces further complications after EU leaders met virtually on Thursday to discuss possible vaccine export bans.
The agenda of the meeting was to give member states greater scope to block vaccines being exported outside the bloc.
The proposal would apply to all vaccines including AstraZeneca, on which the EU had originally been relying to meet a goal of inoculating 70% of its adult population by this summer.
India also put a temporary hold on all major exports of the Anglo-Swedish firm’s vaccine which is produced by the Serum Institute of India (SII), in order to meet domestic demand as infections rise, Reuters cited two sources as saying.
The COVAX vaccine-sharing scheme, backed by the World Health Organization (WHO), warned that the move would delay supplies to dozens of lower-income countries also relying on SII production.
AstraZeneca’s vaccine is seen as crucial in tackling the coronavirus pandemic as it is cheaper and easier to transport than many rival shots.
EU nations have recently started to raise concerns over side-effects and efficacy data of the AstraZeneca vaccine.
Earlier this week, the Netherlands, Ireland, Indonesia, France, Germany and Denmark stopped the use of the vaccine but decided later to lift suspensions as their governments raced to reassure an exhausted and anxious public that it was safe amid a new wave of infections.
The European Medicines Agency said last week that the vaccine was safe and not linked to a rise in overall risk of blood clots.
The EU had initially cast doubt over the vaccine efficacy in the over-65 age group — the most in need of the shot due to age-related coronavirus risks.
A trial data involving older participants, however, showed that the vaccine was safe and effective for the group.
On Monday, the findings of a large US trial showed that the vaccine was safe and highly effective.
But a US health agency cast doubt over published efficacy rates on Tuesday, saying that AstraZeneca may have included “outdated” information in the trial results.
The following day, AstraZeneca issued updated phase three trial data for its vaccine that purportedly showed the jabs were 76% effective.
The drugmaker, which is awaiting US regulatory approval for its shots, also reiterated that the vaccine was 100% effective against severe or critical forms of COVID-19.
“The vaccine efficacy against severe disease, including death, puts the AZ vaccine in the same ballpark as the other vaccines,” said William Schaffner, an infectious disease expert from the Vanderbilt University School of Medicine.
Some European experts believe that the AstraZeneca vaccine could be the victim of nationalism in the US, which is making rival shots.
They also say the AstraZeneca vaccine has become the target of negative sentiment in Europe directed at the UK after its exit from the European Union.
Relentless Israeli ceasefire violations justify need for self-defense: Lebanese MP
Tel Aviv tells Damascus Israeli forces will remain in occupied territory: Report
Dec. 22: ‘Axis of Resistance’ operations against Israeli occupation
‘Abhorrent’: Oxfam says only 12 trucks delivered aid in North Gaza since Oct.
VIDEO | Leader receives religious eulogists on Hazrat Fatima birth anniv.
Pope Francis slams Israel’s ‘machine-gunning’ of Gaza children
US hostage-taking of Iranian nationals violation of intl. law: Deputy FM
VIDEO | Carol Singers for Palestine on London’s Parliament Square












 This makes it easy to access the Press TV website
This makes it easy to access the Press TV website