Iran approves second homemade COVID vaccine for emergency use
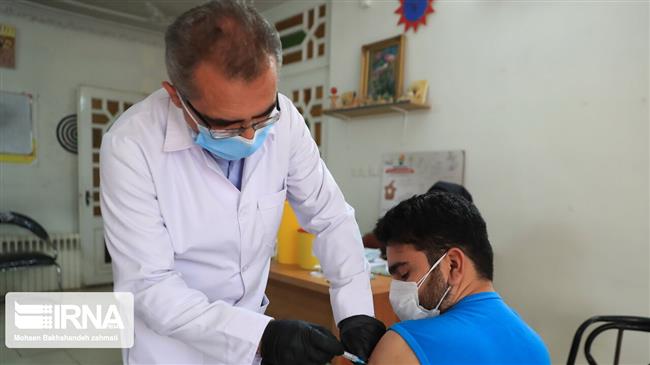
Iranian health ministry has approved a second COVID-19 vaccine developed and manufactured inside the country for emergency use.
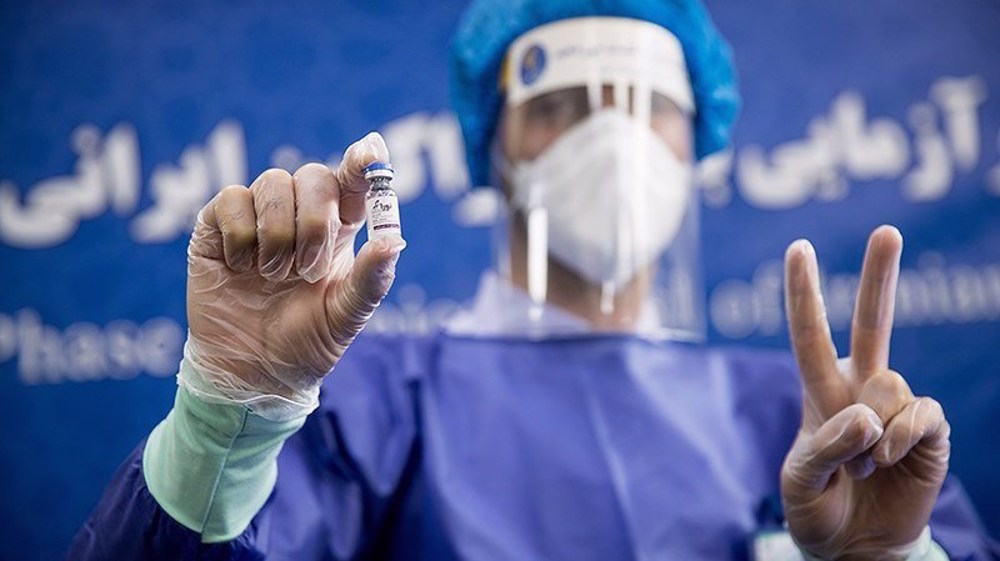
Health minister Saeid Namaki said on Tuesday that the vaccine developed by the Pasteur Institute of Iran had obtained the emergency use approval earlier in the day after successfully completing various trial stages.
“The third phase of the clinical trial for the Pasteur vaccine which is the second Iranian vaccine (against) cornavirus has been carried out and today we were able to obtain the permission for its emergency use form the legal committee,” said Namaki.
Iran had approved its flagship Coviran Barekat vaccine for emergency use earlier this month. The Pasteur vaccine has been developed as part of a joint experiment with Cuban scientists.
The approvals come as the government seeks to accelerate its inoculation program against the coronavirus some six months after it began using supplies from other countries.
The campaign has mostly relied on Russian and Chinese jabs and others provided by a World Health Organization project.
Nearly six million shots have been delivered to the elderly and the frontline health workers in Iran of which nearly one million have gone to people receiving a second dose for better protection against the disease.
Government authorities expect the number of vaccinations would reach 13 million in mid-August.
Iran has three other coronavirus vaccine candidates, including two being developed by scientists in the country’s armed forces and one by the Razi Vaccine and Serum Research Institute, an established pharmaceutical company run by the government.
Facing a series of inhumane sanctions imposed by the United States, the Iranian government has mostly relied on home-grown capacities to tackle one of the harshest outbreaks of the coronavirus in the Middle East.
Health ministry figures published on Tuesday put the daily death toll from the disease and the number of confirmed cases at 142 and 12,717, respectively.

Iran’s steel output up 3.7% y/y to 3.3 million mt in March

Iran’s annual inflation up 0.7% to 33.2% in April: SCI

Iran’s annual dairy exports double to nearly $1 billion
China supports Iran-US indirect talks, defends Tehran's nuclear right
VIDEO | Gaza’s dire conditions hit unprecedented levels
VIDEO | Press TV's news headlines
VIDEO | Pakistan’s business and cultural front unites for Gaza: Nationwide shutdown, boycott announced
US jets carry out more aggression against Yemen
Syrian militants enslaving Alawite women in Idlib governorate: Report
VIDEO | US pro-Palestinian campus protest
VIDEO | Palestinian civil defense rejects Israel’s probe and exposes the crime






 This makes it easy to access the Press TV website
This makes it easy to access the Press TV website