Deputy health minister: Iran, Russia to cooperate in COVID-19 vaccine production
A delegation from Iran’s Health Ministry headed by a deputy health minister has arrived in Moscow to discuss cooperation between the two sides in a variety of health-related areas, particularly the co-production of Russia’s coronavirus vaccine.
The team is set to hold talks with Russian officials, including the health minister, on the co-production of Sputnik-V vaccine, and expects to sign an agreement with the Gamaleya Research Institute of Epidemiology and Microbiology, which develops the vaccine.
The Iranian delegates also plan to visit the vaccine’s production line at a factory outside of Moscow.
Upon arriving in Moscow on Sunday, Iranian Deputy Health Minister Mohammad Reza Shanehsaz told reporters that preliminary talks over the purchase and co-production of Sputnik-V started a few months ago, thanks to the efforts made by Iran’s Embassy in Moscow and the Iranian Foreign Ministry.
“During this visit, we will hold the final round of talks for the co-production of the Russian vaccine,” said Shanehsaz, who is also the head of Iran’s Food and Drug Administration.
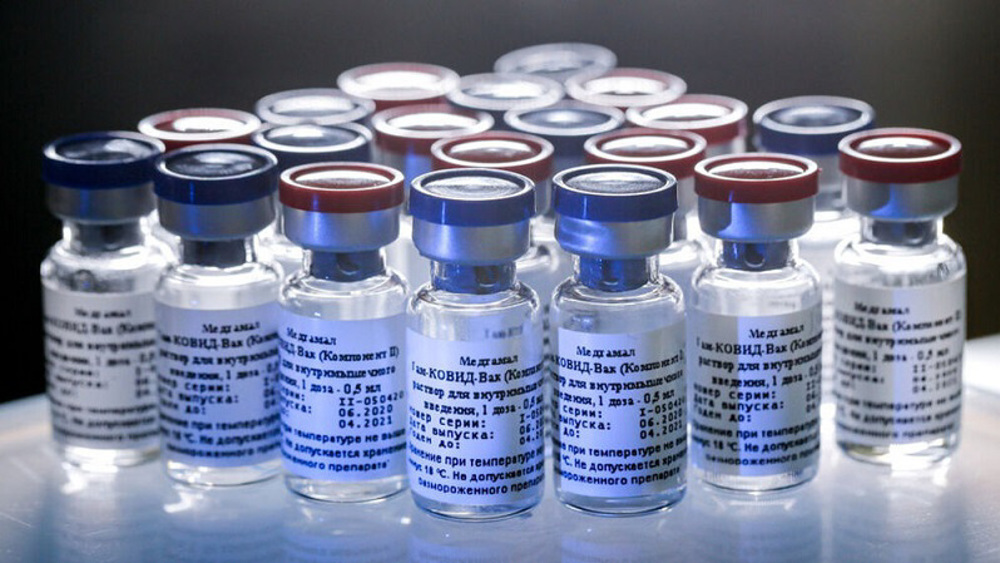
The first batch of Sputnik-V arrived at Iran’s Imam Khomeini International Airport on February 4, days after the medical journal Lancet published an article proving its effectiveness based on trial vaccination of nearly 22,000 volunteers. The results showed a 91.6% efficacy of the vaccine.
Shanehsaz said on the day that Iran had purchased 2 million doses of the vaccine.
Iranian Ambassador to Moscow Kazem Jalali had earlier said the next shipments would arrive in Iran in the upcoming weeks and months.
Iran also signed an agreement with Cuba last month to cooperate in the production of a vaccine called Subrana 2, which is now undergoing the human phase trials by the Cuban Finlay Institute and the Pasteur Institute of Iran.
Iran has also undertaken major efforts – in the midst of US sanctions – to develop two homegrown vaccines called Coviran Barekat and Razi COV-Pars, which were produced by the Headquarters for Executing the Order of Imam Khomeini and the Razi Vaccine and Serum Research Institute respectively.
The new development came after earlier the same day, Iran launched the first phase in the human trial of its second COVID-19 vaccine developed by the Razi Vaccine and Serum Research Institute.
The vaccine, Razi COV-Pars, was unveiled during a ceremony in Tehran, with Health Minister Saeed Namaki, Agriculture Minister Kazem Khavazi and Vice President for Science and Technology Sourena Sattari in attendance.
Razi COV-Pars, administered via injection and inhalation, is an mRNA recombinant vaccine that reconstructs a harmless piece of the virus’ spike protein.
Tel Aviv tells Damascus Israeli forces will remain in occupied territory: Report
Dec. 22: ‘Axis of Resistance’ operations against Israeli occupation
‘Abhorrent’: Oxfam says only 12 trucks delivered aid in North Gaza since Oct.
VIDEO | Leader receives religious eulogists on Hazrat Fatima birth anniv.
Pope Francis slams Israel’s ‘machine-gunning’ of Gaza children
US hostage-taking of Iranian nationals violation of intl. law: Deputy FM
VIDEO | Carol Singers for Palestine on London’s Parliament Square
Ansarullah says ‘Israeli terrorists’ incapable of confronting Yemen, warns of secret weapons
















 This makes it easy to access the Press TV website
This makes it easy to access the Press TV website