Russia says Sputnik V virus vaccine 95% effective
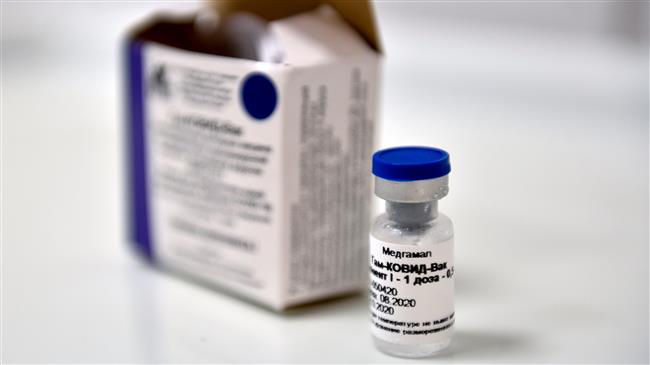
Russia said Tuesday its Sputnik V coronavirus vaccine was 95 percent effective and would be cheaper and easier to store than some alternatives, as the global race heats up to develop a jab.
The announcement was the latest in a flurry of breakthroughs as several vaccine makers worldwide published preliminary data showing efficacy rates of 90 percent and higher.
Countries are hoping to begin inoculating their populations by year's end or in early 2021 to stop a pandemic that has claimed the lives of nearly 1.4 million people.
Russia was one of the first to announce the development of a vaccine in August — dubbed Sputnik V after the Soviet-era satellite — but before the start of final clinical trials.
In statement on Tuesday, the vaccine's developers said preliminary data after trials involving thousands of volunteers showed "an efficacy of the vaccine above 95 percent" after a second dose.
Russia's health ministry, the state-run Gamaleya research center and the Russian Direct Investment Fund (RDIF) said in the statement they expected the vaccine to record an even higher effectiveness after the next analysis.
"No unexpected adverse events were identified as part of the research," it said, though some of those vaccinated suffered short-term effects including fever, weakness, fatigue, and headache.
Less than $10 per dose
The two-dose vaccine will be available on international markets for less than $10 per dose, they said, and will be free for Russian citizens.
It can be stored at between two and eight degrees Celsius (between 35.6 and 46.4 degrees Fahrenheit), they said, instead of the temperatures far below freezing required for some other vaccines.
Pharma giants Pfizer and BioNTech announced that their virus vaccine is 95 percent effective, while US company Moderna said last week early results showed its candidate was 94.5 percent effective.
Western experts have in the past expressed concern over Russia's vaccine, fearing that its development could be rushed.
Several welcomed Tuesday's announcement though they said more data was needed.
"This is yet more good news demonstrating a high interim vaccine efficacy," said Azra Ghani, an infectious diseases specialist at Imperial College London.
"The lack of any serious adverse events in approximately 20,000 trial participants is also very encouraging," she said.
Russia has applied to the World Health Organization for accelerated registration and pre-qualification of the Sputnik V vaccine.
President Vladimir Putin last week said that Russia had manufacturing agreements in place with China and India and encouraged Brazil and South Africa to also mass produce Russian-developed vaccines.
The head of the RDIF, Kirill Dmitriev, said Tuesday that European Union member Hungary would also manufacture the Sputnik V vaccine.
Russia in recent weeks has registered a steep increase in new coronavirus infections but has stopped short of introducing strict measures like some European countries.
'Alarming' rise in Russia cases
The country's total infections on Tuesday stood at 2.14 million, the fifth-highest caseload in the world.
But at 37,031, virus deaths are significantly lower than other badly hit countries, raising concerns that the authorities are downplaying the pandemic's severity.
Data published by Russia's federal statistics service earlier this month indicated excess deaths of more than 117,000 year-on-year between March and September, suggesting that virus fatalities could be much higher.
While Moscow remains the epicenter of Russia's outbreak, the country's poorly funded regions are taking the brunt of the second wave.
Deputy Prime Minister Tatiana Golikova on Tuesday said that in six of Russia's 85 regions, over 90 percent of hospital beds dedicated to Covid-19 patients were occupied.
Russia hopes to avoid a second nationwide lockdown by accelerating the production of vaccines.
Putin has dismissed talk of another lockdown and emphasized last week that vaccines are on their way despite voicing concern over the country's "alarming" case totals and fatality rate.
He last month announced that Russia had registered a second coronavirus vaccine, EpiVacCorona.
Kremlin spokesman Dmitry Peskov on Tuesday said that Putin, who has said that one of his daughters took part in the Sputnik V clinical trials, could not volunteer to take the vaccine as "head of state.”
(Source: AFP)

Putin orders emergency planes to Iran after blast at port of Bandar Abbas

Ukraine may have to 'give up land' to Russia to secure peace: Kiev mayor

Trump Ukraine mediation muddle
VIDEO | Press TV's news headlines
Iran more than halved its power grid losses to 10% in 16 years: Expert
Abbas names likely successor in move deemed ‘illegitimate, divisive’
Illegal Israeli settlers attack Palestinian school in occupied West Bank
VIDEO | Israeli forces tighten siege on Jenin refugee camp
Iran restores operations at its largest container port after explosion
Leader offers condolences over ‘painful’ port blast in southern Iran
VIDEO | Yemeni army attacks sensitive Israeli military sites with ballistic missiles
















 This makes it easy to access the Press TV website
This makes it easy to access the Press TV website